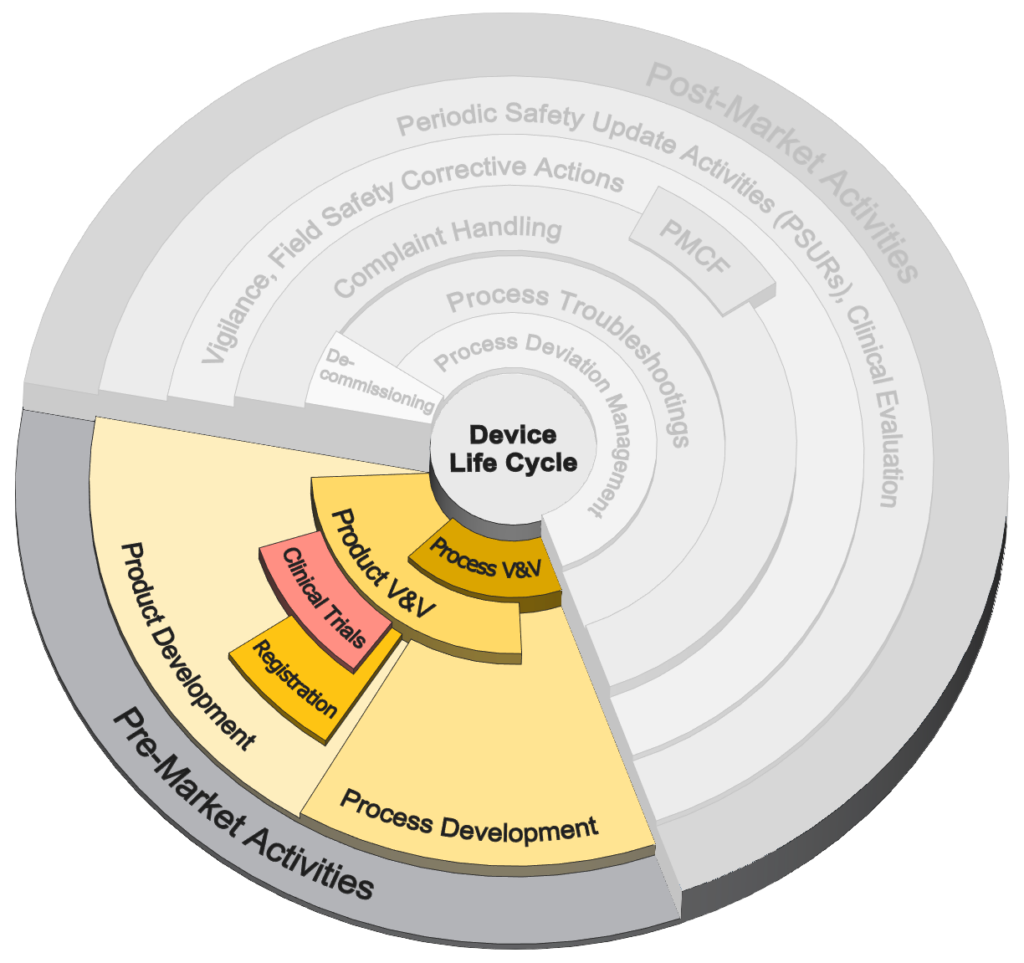
Pre-Market Activities
Selection of services offered for activities before commercial launch.
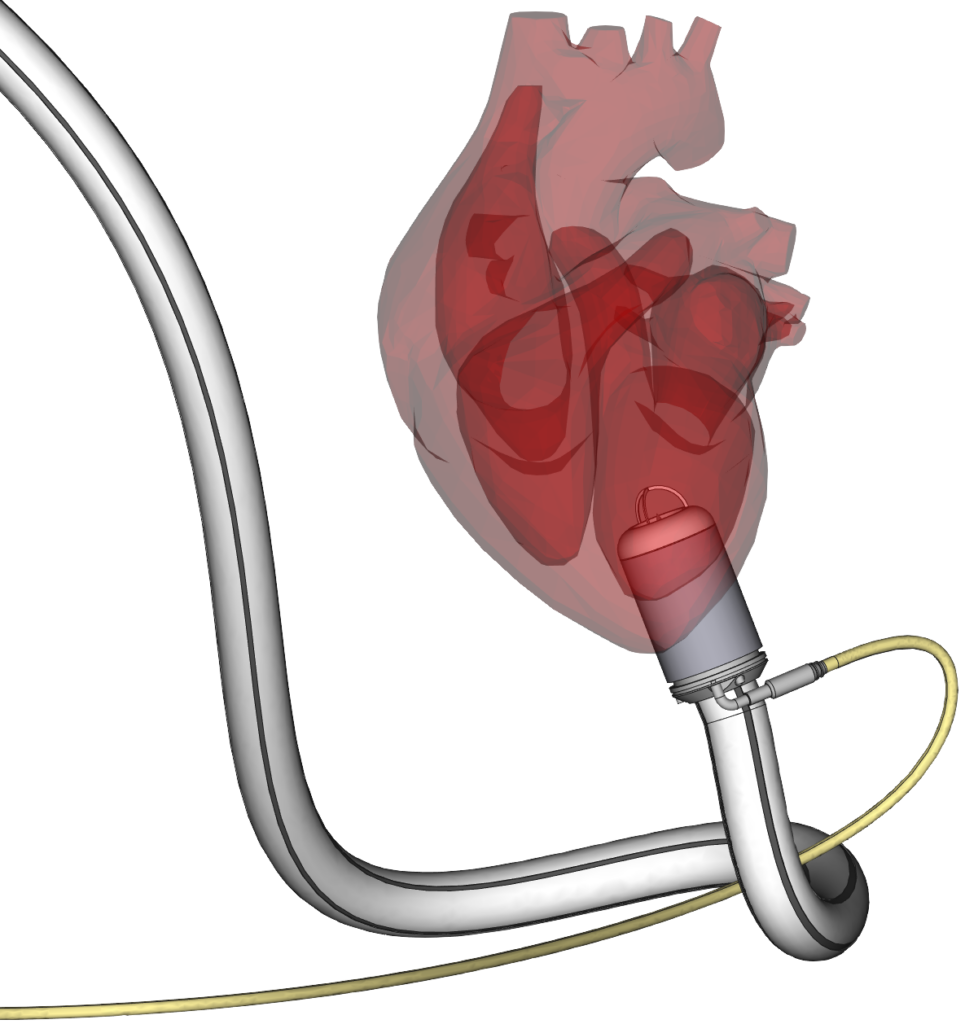
Product Development
• CAD Designs and Simulations
• Design for Manufacturing (low cost production)
• Medical Devices
• Industrial / Commercial Products
Verification / Validation
• Statistical analysis and root cause analysis
• Prototyping and unit testing
• Bio-compatibility
• Certification (e.g. electrical)

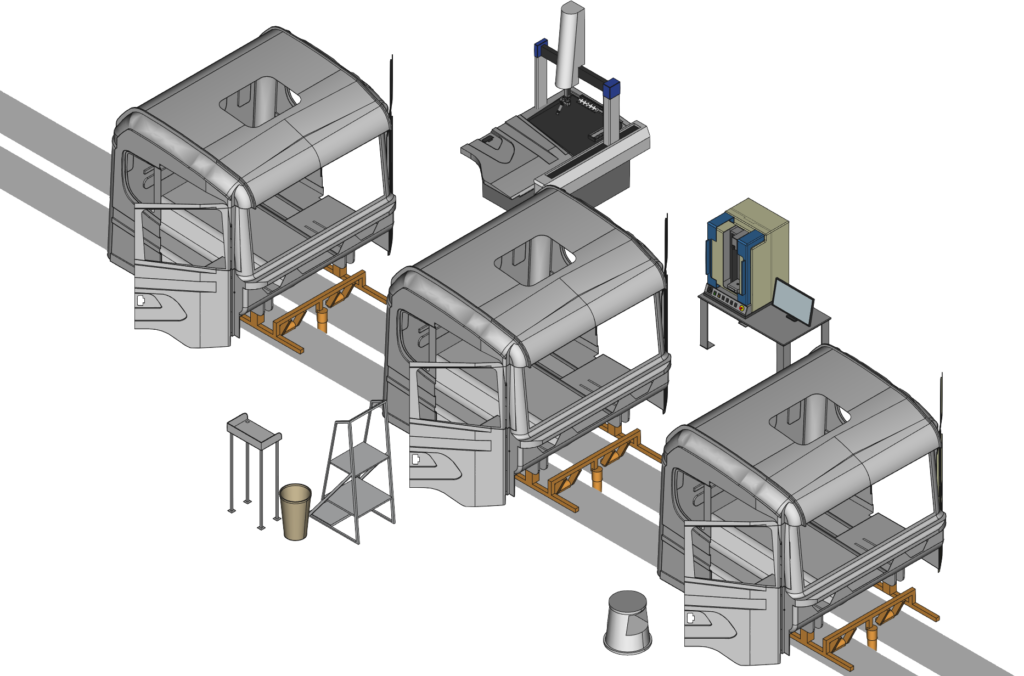
Process Development
• Conception
• Investment Analysis
• Simulations
• Capacity Analysis
• Flow and Lead Time Analysis
• In-Process Quality Control
• Setup of process monitoring and feedback systems
Regulated Process Controls
• Clean-room setup and qualification
• Medical Device / Pharmaceutical applications
• Validation and Verification per regulatory requirements
• Environmental Controls

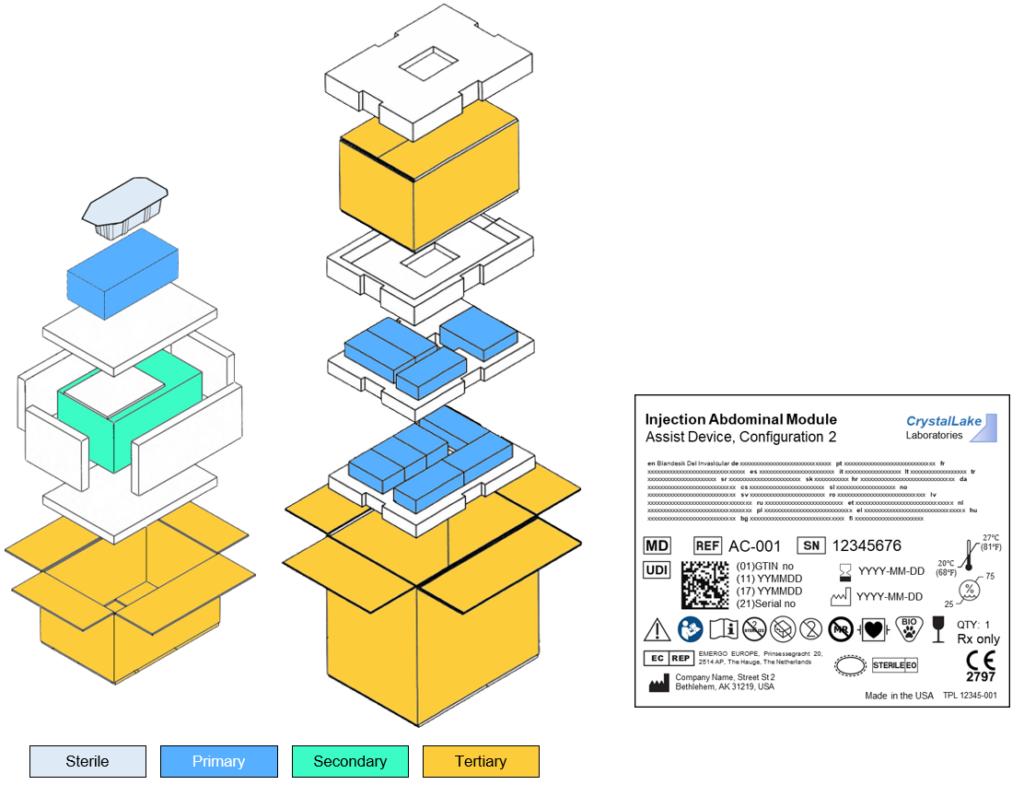
Packaging and Labeling
• Design / Development
• Testing, Validation and Verification
• Packaging, storing, transporting;
- Sterile
- Biological
- Li-ion
- Electronic

Equipment Setup and Qualification
• CNC milling
• Ceramic, Metallic (incl. Super Alloys), Acrylic / Polymers
• Plastic Injection Molding
• CMM
• Visual, Light and Touch probing
• Sintering, Anodization
• Packaging and Distribution
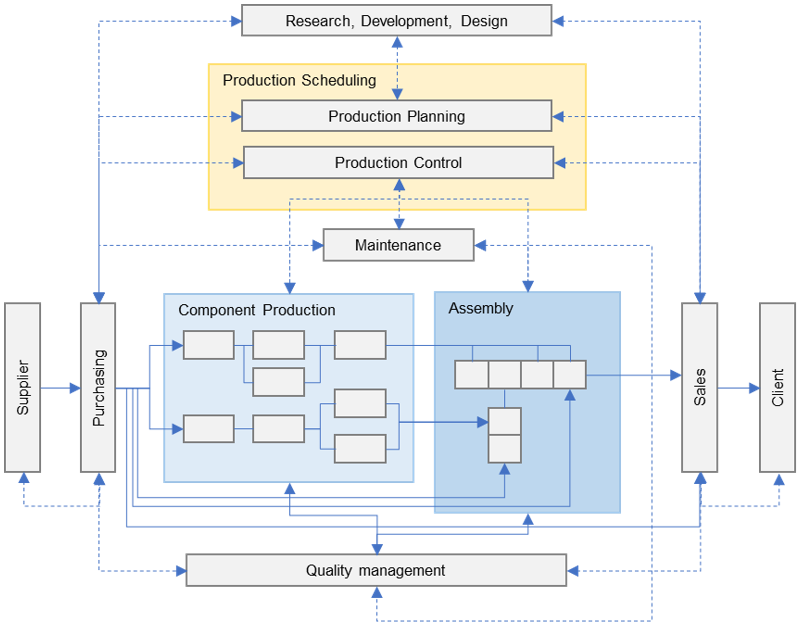
Process Flow Analysis
• Production Scheduling
• Waste eliminations
• Variation Reduction
• Standardization & simplifications
• Capacity and capability optimizations
• Risk Mitigations
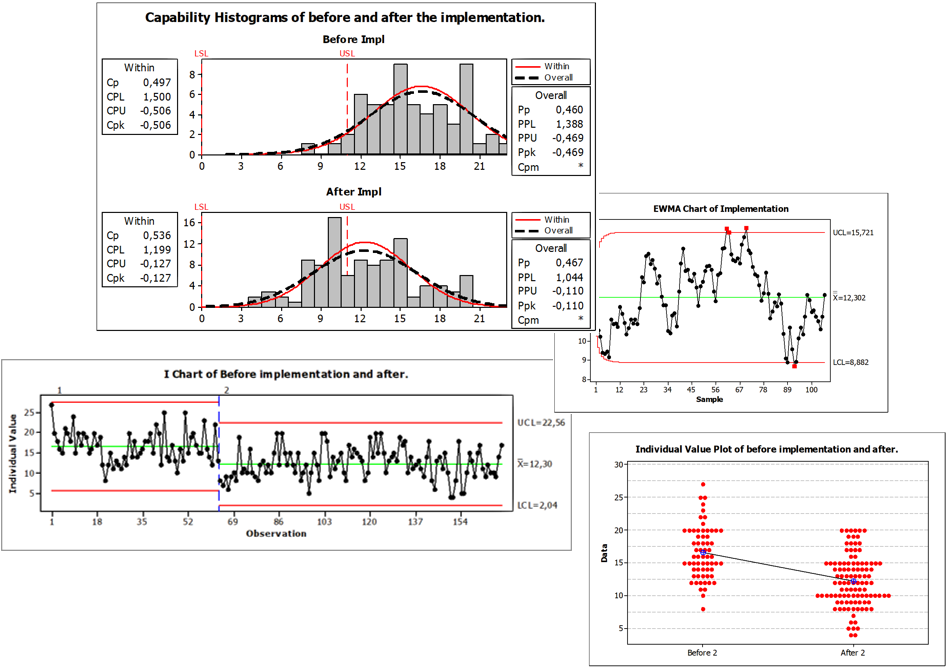
Performance Analysis
• Process Capability Analysis
• Effectiveness and Efficiency Analysis
• Trend Analysis
• Root Cause Analysis
• Design-of-Experiments
• MSA (Gage R&R)
