Post-Market Activities
Selection of services offered for activities after commercial launch.
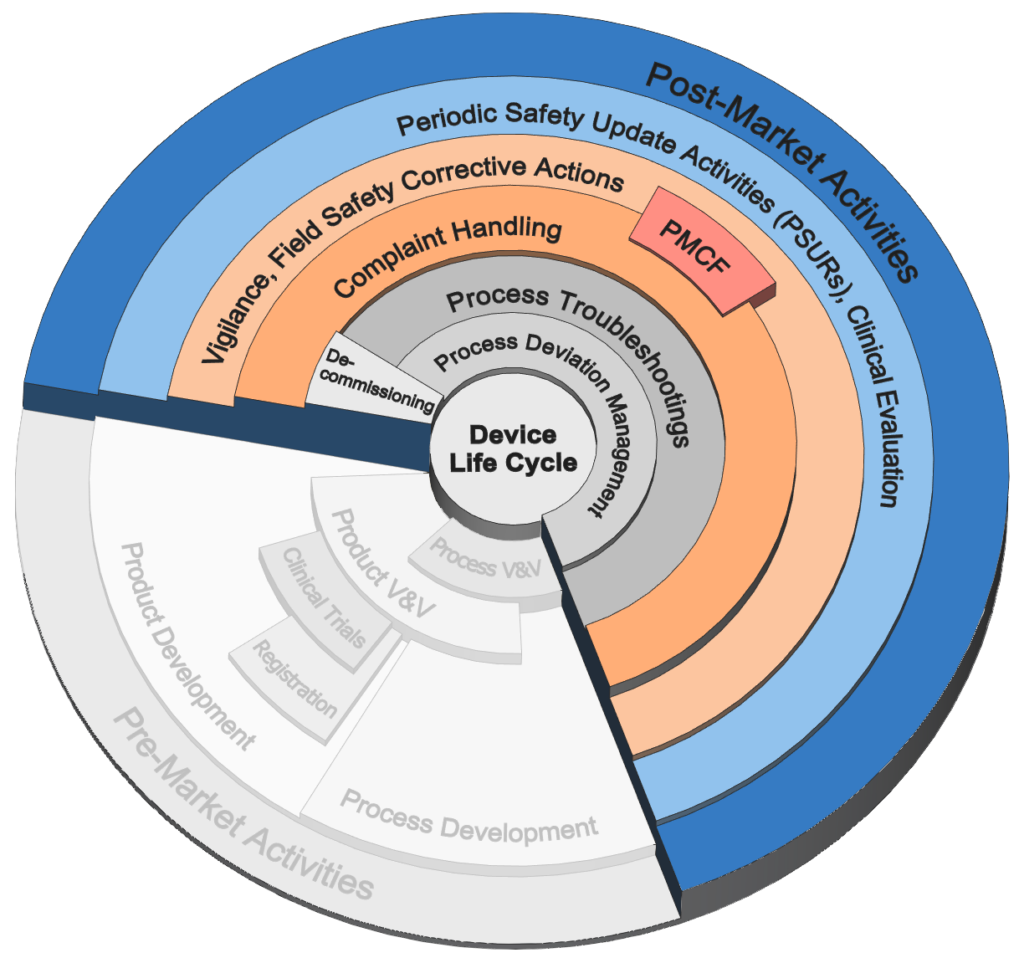
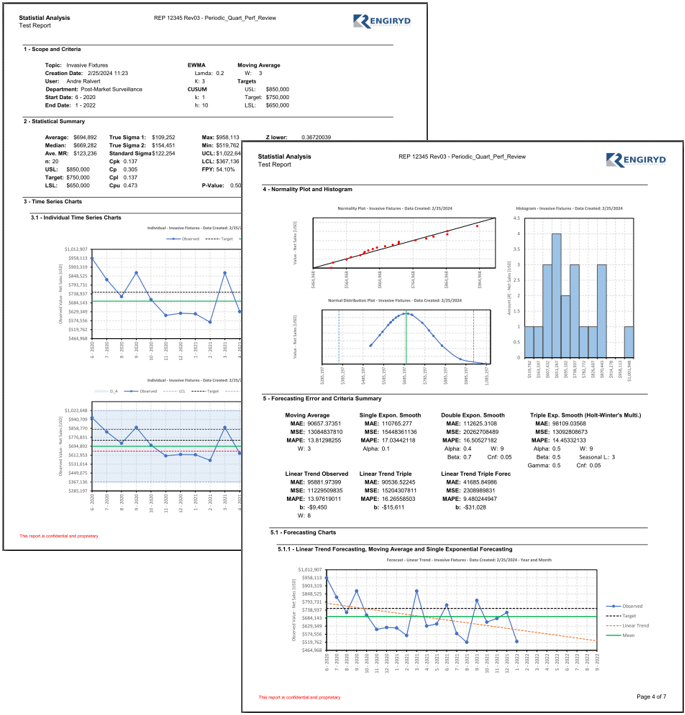
Periodic Safety Updates
• Periodic Safety Update Reports (PSURs)
• Data gathering and Analysis
• Trending
• Competitor Analysis
• Proactive Surveys
• Benefit / Risk Analysis
• Summary of Safety and Clinical Performance
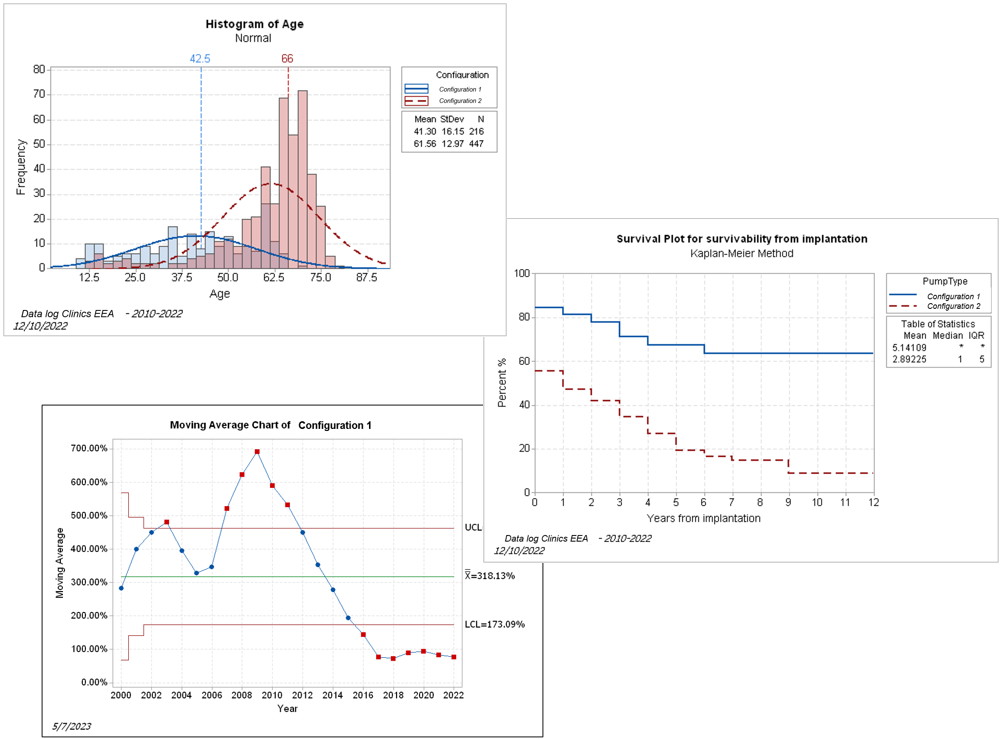
Clinical Evaluation
• Clinical Investigation and Reporting
• State-of-art justification
• Data analysis
• Literature review
• Benefit / Risk Analysis
• Update of IFUs and regulatory files
Quality Management System
• Maintenance and Compliance updates
• FDA 21 CFR Part 820, EU MDR 2017/745
• ISO 13485, ISO 14971, ISO 9000
• Efficiency / Productivity Improvements
• Simplification
• Waste eliminations
• Organization / Structurization

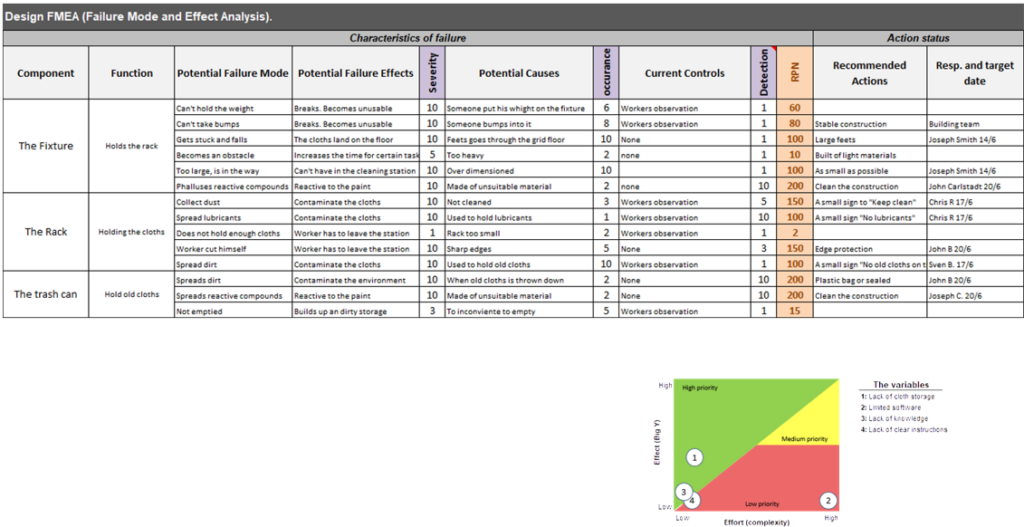
Risk Management
• Rapid updates and simplification of risk files
• Risk assessments and mitigations
Vigilance Handling
• Health Hazard Evaluations
• Product / Process Root Cause Analysis
• CAPAs
• Global Field Actions
• Recalls / Services
• Safety notices
• Coordination with authorities
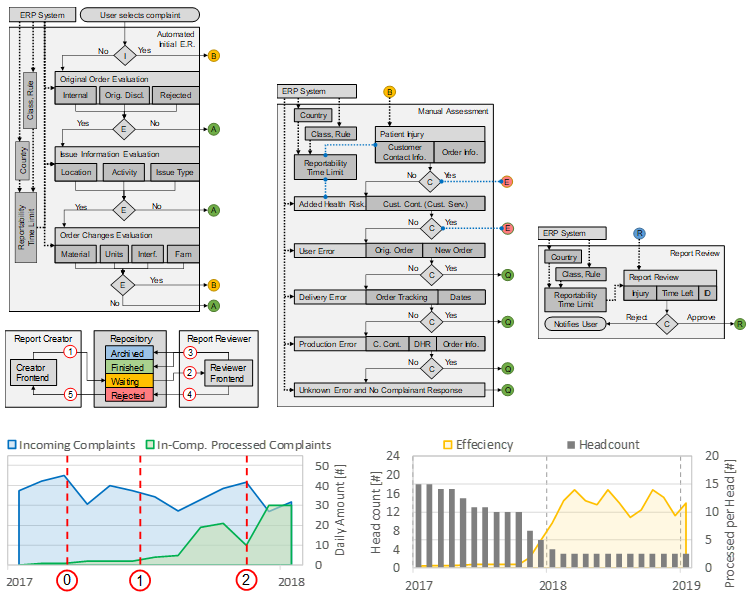
Complaint Handling
• Complete Process Development and Deployment
• Medical Device Reporting
• Complaint Investigations and Field Investigations
Process Deviation Management
• CAPA management (Corrective and Preventive Actions)
• Non-conformance material handling
• Rapid production recovery and supply chain continuation
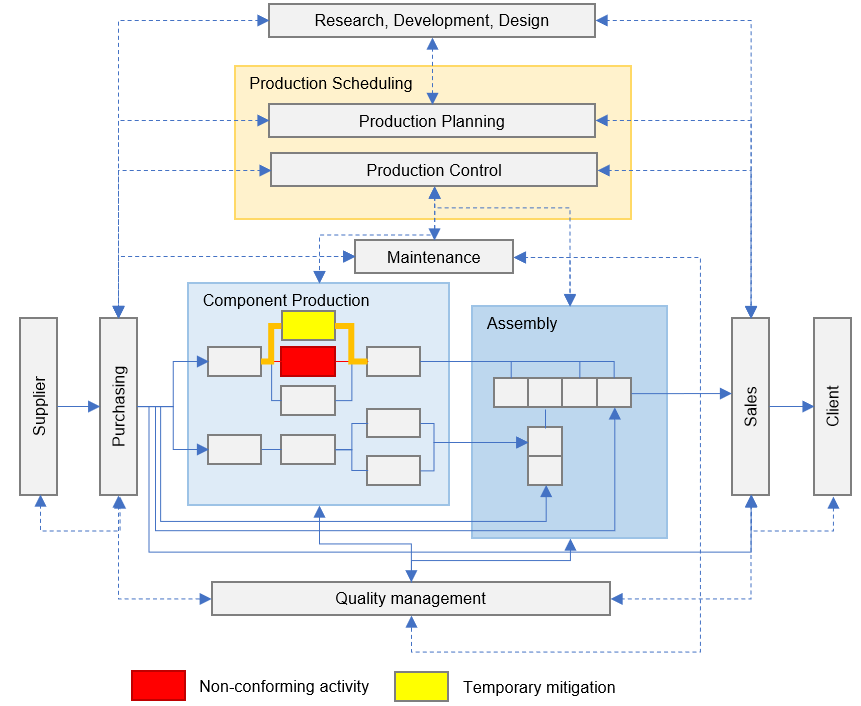
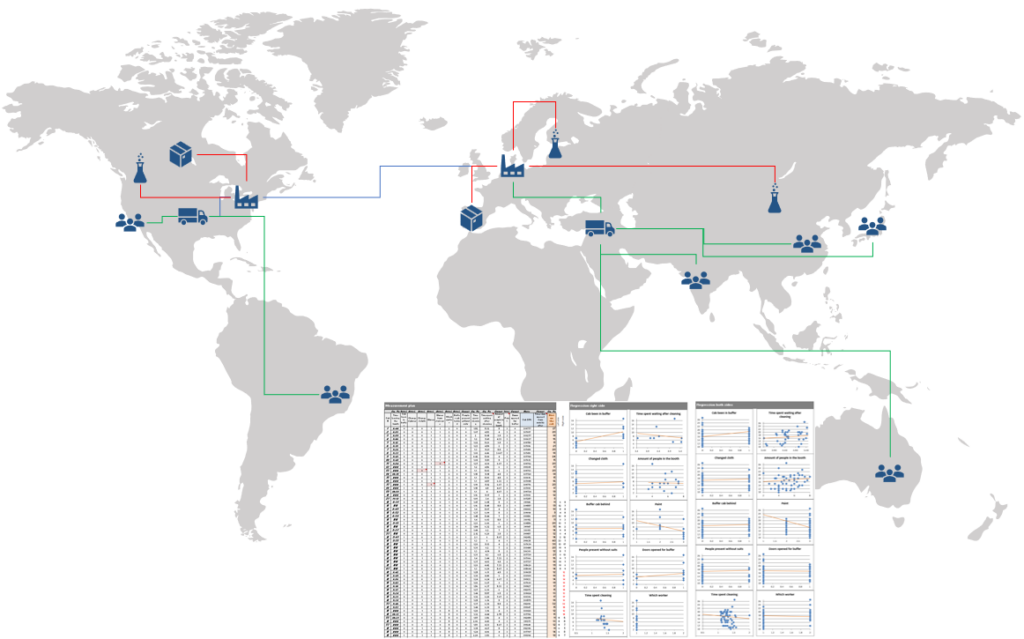
Supply Chain Troubleshooting
• Analysis and rapid recovery of global supply chains issues
• Immediate organizing multi-functional and globally disperse teams/functions
