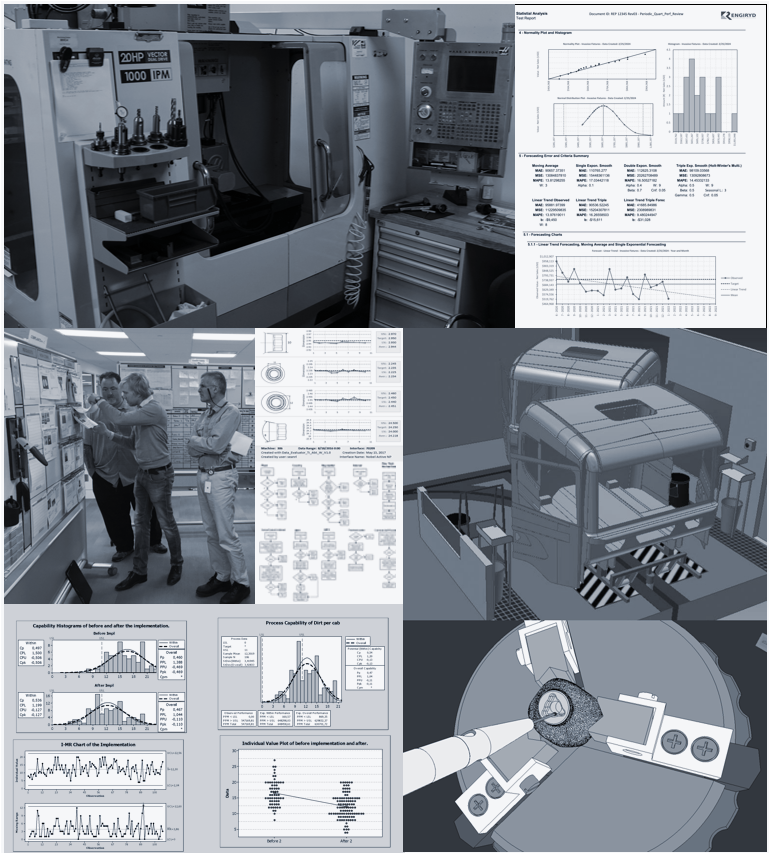
Engineering Services
Contracting and consulting services covering from local to global mass-manufacturing environments, global supply chains and R&D.
+10 years
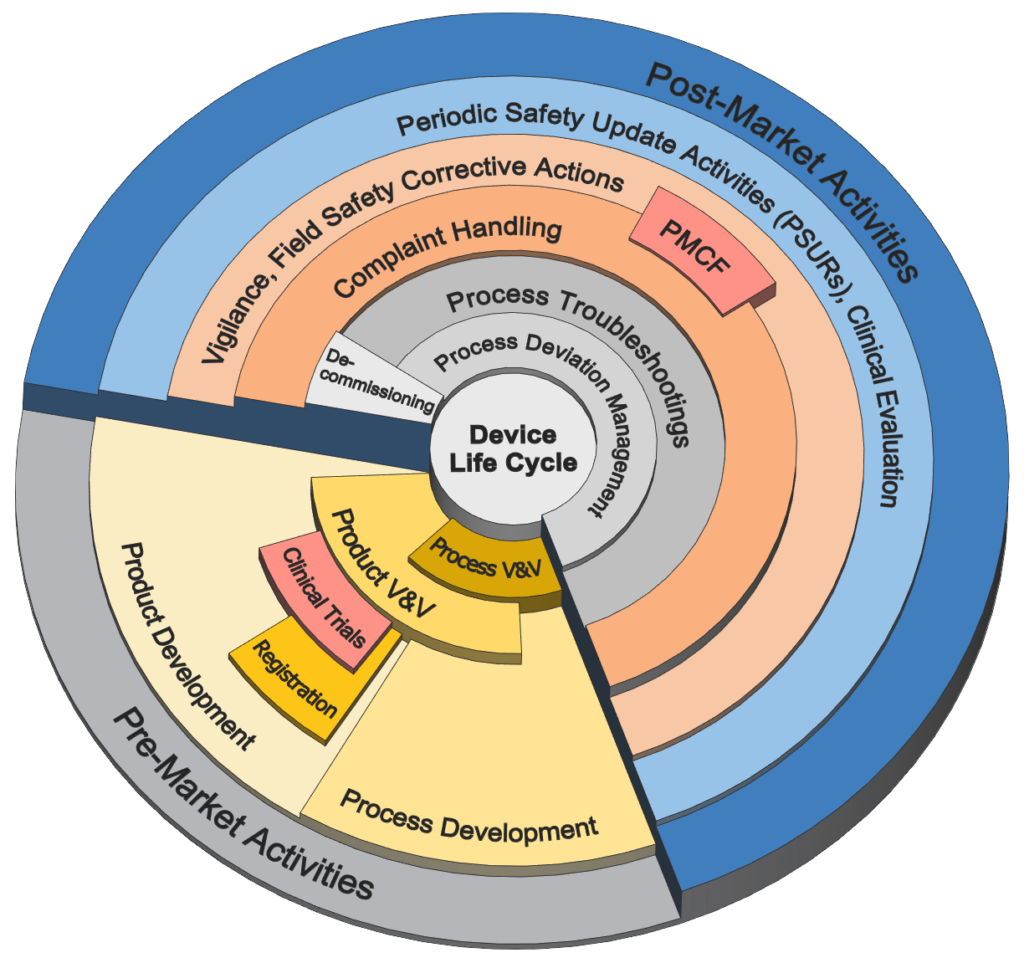
Medical Device
Life Cycle Activities
+9 years
Maintaining
Global Supply Chains
+8 years
Lean Six Sigma
Implementation
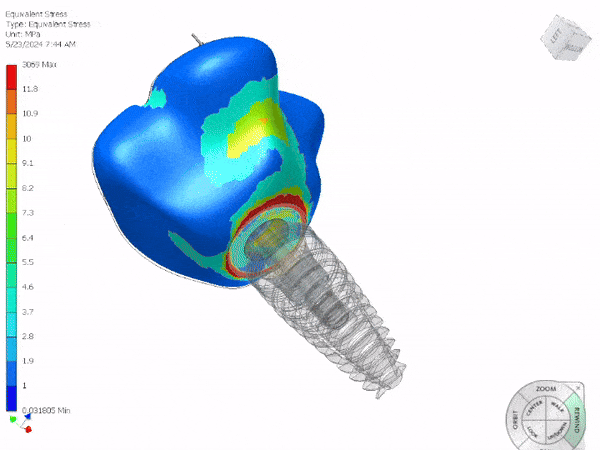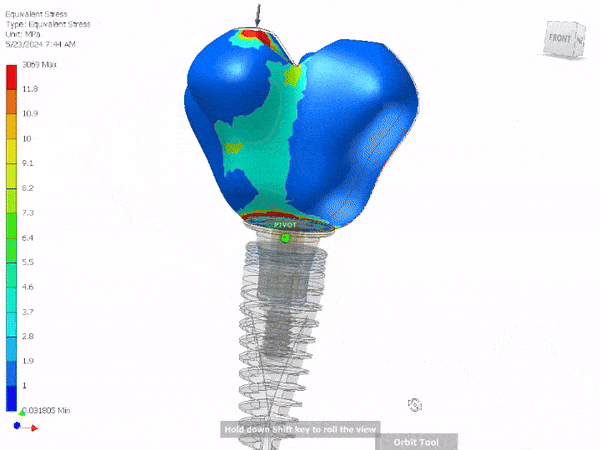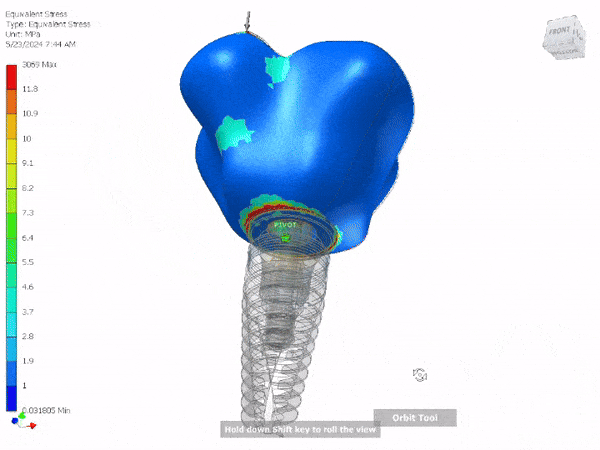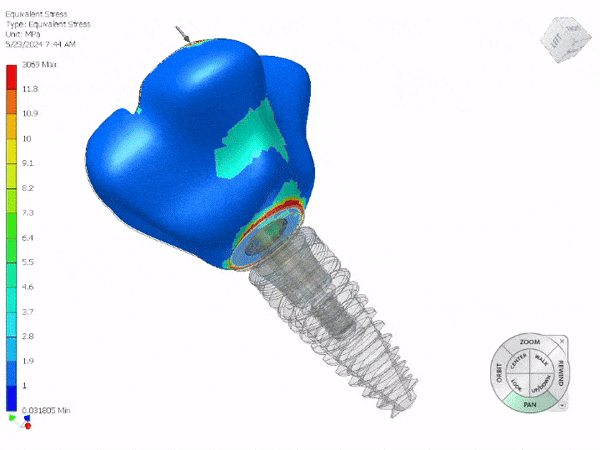
Entire Life Cycle
- Heavily Regulated Industries
- Medical Devices
- Pharmaceutical
- Automotive
- Production, Products and Services
- Complete Life Cycle Support
Our Services
- Complete coverage of the entire device/service life cycle
- Engineering Activities (see tabs for further details);
- Pre-Market Activities
- Post-Market Activities
- Device Development, Service, and Troubleshooting
- Regulatory Activities;
- Device / Service Commercial Approvals
- Deviation Management

Covered Regulatory Frameworks
- EU MDR 2017/745
- EU AI-MDD / EU MDD
- FDA 21 CFR Part 820
Devices Classes and Types
- [EU MDR 2017/745]:
- Invasive and Permanent
- Class I/s/r/m/mr, IIa, IIb, III
- Passive and Active
- Restorative and Life Supporting
- Hardware and Software